| Adiabatic Lapse Rate | |
|
|
|
|
If we climb to altitude we get closer to the sun. So why do we need warm clothes on top of Everest? Imagine a parcel of air molecules with an imaginary boundary around them. Move this parcel upwards-it will experience less pressure and vice versa. If it expands (as the pressure decreases) work must be done as the molecules move apart, this work or energy is in the form of heat thus the parcel of air will cool. Thus we can see why we should expect parcels of air that have moved to higher altitudes (and thus expanded) to be cooler. |
 |
|
Under ideal conditions, on an overcast day with the air perfectly dry, the rate of cooling is around 1oC per 100m of altitude rise. The change in temperature with altitude is called the lapse rate and the theoretical ideal we call the dry adiabatic lapse rate. Let us have a brief physics lesson to consider why this is the case. |
|
|
DETERMINING THE ADIABATIC LAPSE RATE We need to establish an equation that describes the relationship between the temperature and pressure of an air parcel as it moves up and down in the atmosphere. We make the assumption that any changes we make at any particular time are very small. The ideal gas law and first law of thermodynamics can be combined to give the following equation: |
|
| dQ=CpdT-VdP | (1) |
|
Where dQ is the quantity of heat added to parcel per unit mass of air (J/kg) Cp is the specific heat of air (at constant pressure.) J/kg.oC dT is the incremental temperature change (K) V is the volume per unit mass of air (m3/kg) And dP is the Incremental pressure change in the parcel (Pa). Assume that, as the parcel moves, there is no heat transferred across the boundary, that is, that this process is adiabatic. This means that no heat is added to or taken from the parcel i.e dQ=0 so we can rearrange equation (1) above as: |
|
| dT/dP= V/Cp | (2) |
|
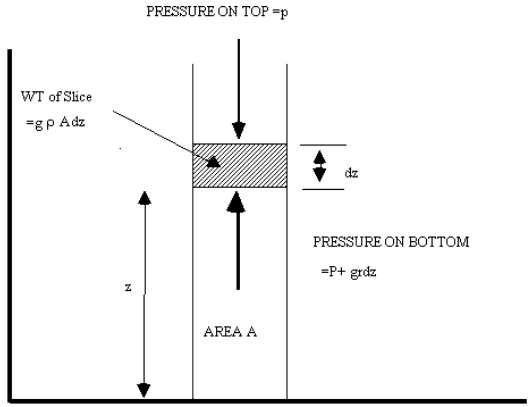
This gives us an indication of how atmospheric temperature changes with air pressure but what we need to know is how it changes with altitude. Consider a static column of air with cross section A A horizontal slice of air in this column with thickness dz and density |
|
 |
|
|
If the pressure at the top of the slice = P(z + dz) Then pressure at the bottom of slice P(z) will be P(z + dz) plus the added weight per unit area of the slice itself. |
|
| (3) | |
|
Where g is the gravitational constant We can write the incremental change in pressure with altitude dP as: |
|
| (4) | |
|
If we now express the rate of change in temperature with altitude dT/dz. as the product (dT/dP)(dP/dz) and substitute equations (2) and (4) into this product we see: |
|
| (5) | |
|
However Since V = volume per unit mass and |
|
| dT/dz= -g/Cp | (6) |
|
The negative sign indicates that temperature decreases with altitude. If we substitute for g = 9.806 m/s2 and the specific heat for dry air at room temperature , Cp = 1005 J/kgoC and substitute for the joule in terms of kg m2/s2 equation (6) tells us that : dT/dz = (9.806 m/s2 /1005 J/kg oC)x 1J/ kg m2/s2 = -0.00976oC/m We normally get rid of the negative sign and express -dT/dz as 9.76oC/km. This is normally given the symbol In effect REAL CONDITIONSIn our discussion on the dry adiabatic lapse rate we assumed that the air was dry. The specific heat of the gas is clearly important in fixing the lapse rate and a specific correction for water vapour may be necessary if air is very wet. If air is saturated the situation is complicated by the release of latent heat when water condenses. The wet adiabatic lapse rate is not a constant but a good average is about 6oC/km. However for our purposes we will continue to treat the air as dry. WHY IS THE LAPSE RATE IMPORTANT TO US? |
|
| The reason why an understanding of the lapse rate is important is that the lapse rate can be used as a measure of air stability and thus to indicate how well pollutants will be dispersed. | |
|
If lapse rate is greater than predicted by theory the air is unstable and mixing of pollutants takes place. If the lapse rate is less than predicted the air is stable and pollutants are not dispersed. |
|
 is about 1 oC/100m
is about 1 oC/100m