| Global Warming and the Car |
|
Burning fossil fuel also produces carbon dioxide (CO2), probably the best known "greenhouse gas". A small amount of CO2 is absolutely necessary for life on earth, plants produce and absorb it during their lifetime as part of the photosynthesis process, (through which plants "breathe" and produce food). However an excess of CO2 is thought to be the main reason why global warming is occurring. Cars have in the past also used Chlorofluorcarbons, CFC's in their air conditioning units and CFC's are damaging to the Ozone layer. |
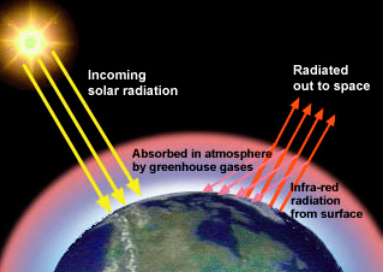
The Greenhouse effect |
 |
|
The basic idea behind this is that short wavelength, high-energy radiation travels to the atmosphere where some is reflected and some is absorbed. A proportion of the radiation heats up the atmosphere and some heats up the surface of the planet. In heating up the surface of the planet the energy is reduced, it is being used to heat the sea and land. As the planet warms up in turn it produces lower-energy longer wavelength radiation (infrared, or heat). This passes up into the atmosphere but, instead of going off into space a large proportion is absorbed by the atmosphere and reflected back to the surface. This occurs because although "green house" gasses in the atmosphere let high energy short wave length radiation pass through them, they trap or reflect the lower energy longer wavelength radiation. Therefore the more "greenhouse gasses" we have in the upper atmosphere the more heat will remain in our system and the earth will progressively heat up. The reason that we call certain gasses "greenhouse" gasses is because they act like the glass in a greenhouse, allowing the solar radiation in but not the long wave heat radiation out. Even in the winter a greenhouse can become quite warm when the sun is shining and this of course is why many gardeners use them to protect seedlings and young plants. |
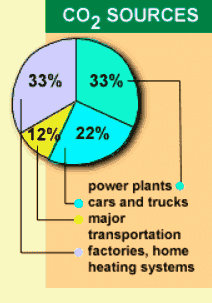 |
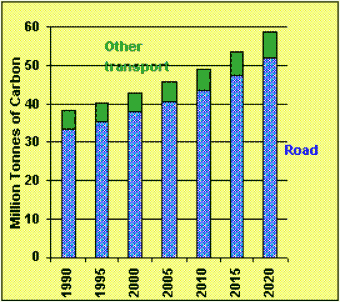
According to the US department of energy, manmade additions to CO2 account for approximately 5-7% of the total global CO2 and transport accounts for approximately 34% of that portion. As more countries become "mobile" this is likely to increase. CO2 concentration in the atmosphere has increased by 30% since the mid 19th century and is currently increasing at approximately 2% per year. In the UK the government have estimated increases in carbon production as part of the process of producing and integrated transport policy (figure 3). The new white paper entitled "A New Deal for Transport: Better for everyone" deals with a variety of methods for reducing car use. |
| Figure 2 CO2 sources (US DOE) | |
 |
|
| Figure 3 Estimated increase in Carbon production in the UK. |
Cars: The most popular way to get around.All current sources suggest that car use is increasing and will continue to do so for some time. Information gathered by the Department of Environment Transport and Regions indicates that not only do we use cars in preference to other forms of transport, but the number of households owning more than one vehicle is also increasing. This increase in the use of cars is also reflected across Europe (figure 5). Figure 6 illustrates NOx emissions, which are estimated to decrease since these are closely related to the production of CO2 you might ask how these emissions will decrease when carbon production is set to increase. There are three main ways to reduce vehicle emissions:
The current reducing trend for production of oxides of nitrogen reflects technical improvements in vehicles but is expected to be reversed eventually as increased levels of traffic outweigh these improvements. |
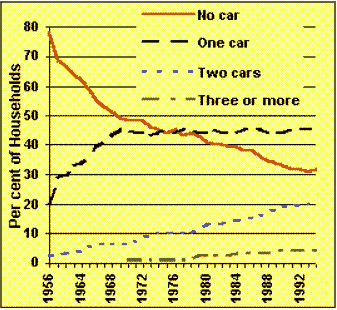 |
Figure 4 Vehicles per household in the UK |
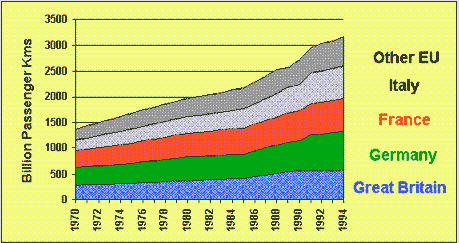 |
| Figure 5 European passengers travelling by road. |
|
CO and NOx are antagonistic, that is, CO is produced in incomplete burning conditions, where temperature and oxygen concentration is low. At higher temperatures with adequate oxygen the CO is converted to the less toxic CO2, unfortunately this high temperature, high oxygen concentration is ideal for the production of the unwanted NOx's. Post-combustion control is fairly easy and economical, sometimes after burners may be used and exhaust re-circulators, where the exhaust fumes are burnt further, however whilst this is good for CO and VOC's it unfortunately provides ideal conditions for the production of unwanted NOx's. For this reason catalytic converters have become more popular. This two step process, (although more expensive), allows NOx's to be reduced to pure Nitrogen, (N2) in the first stage and CO and VOC's to be fully oxidised to CO2 and water in the second stage. |
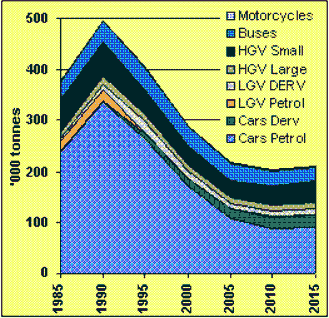 |
Figure 6 UK Nitrogen Oxide emissions by transport type estimated to 2015 |