| Greenhouse and Ozone Depleting Gases |
|
The greenhouse gases occur from natural events and from human activities (anthropogenic), and although there have often been variations to the amounts of naturally produced gases, the concern now is the increases recorded in the last column. It is now widely acknowledged that the increase in these gases has lead to an 'enhanced Greenhouse Effect' and that this is significantly changing the Earth's climate, largely manifested by 'global warming'. The ozone depleting gases are entirely due to our industrial development. These were initially found to be ideal as the working fluid in refrigerators and as the method of blowing foams. ChloroFluoroCarbons CFC's CFC's are strong greenhouse gases and so contribute to global warming, but they are also strong ozone depleters and so contribute to global cooling and it is estimated that these global warming and cooling potentials more or less cancel out. CFC's have been used for a wide variety of industrial applications, such as degreasing, lubricating / cutting, cleaning solvents, in-product solvents for the control of properties such as viscosity, propellants and foam producers and as the working fluid in refrigerators, freezers, air conditioning units and heat pumps. They generally have a long survival time in the upper atmosphere and so are a matter of great concern. HydroChloroFluoroCarbons HCFC's and HydroFluoroCarbons HFC's HCFCs and HFCs contain hydrogen in the structure and have short atmospheric lifetimes and tend to be destroyed in the lower atmosphere by natural processes. HFCs contain no chlorine and therefore do not contribute to ozone depletion, whereas HCFcs contain relatively small amounts of chlorine which provides some ozone depletion. HCFCs are also greenhouse gases. Substitution HCFC-123 could replace CFC-12 in refrigeration and air conditioning plant.
Methane CH4 Methane is an atmospheric trace gas involved in many chemical reactions in the troposphere and stratosphere. Although it is present in much lower concentrations than carbon dioxide each molecule has 11 times the global warming potential of a carbon dioxide. Wetlands, termites and the oceans produce about 150 Tg of methane naturally each year. Anthropogenic releases including about 100 Tg from coal and gas mining, 60 Tg from rice paddies and 80 Tg from enteric animals amount to about 360 Tg per year. |
|
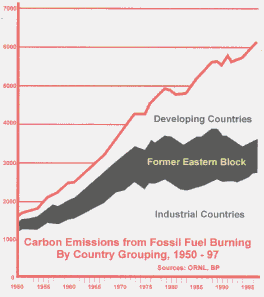
Carbon Dioxide Carbon Dioxide is one of the main greenhouse gases, and is of greatest concern as a controllable gas emission caused by human activities. |
|
|
Burning fossil fuels (coal, oil and gas) to produce energy is the main source of thermal, electrical and motive power in the industrialised countries. Every kilogram of fossil fuel consumed releases around three kilograms of carbon dioxide, which means that the mount of carbon dioxide released in Europe is around 10-15 tonnes per person, per year. In the USA it is more like 25 tonnes per person, per year, accounting for 24 percent of global emissions. The rainforests have always played the role of carbon dioxide absorber. During the 20th Century there has been deforestation on a massive scale, which has meant that much more carbon dioxide now remains in the atmosphere than at any time in recent history. |
|
Nitrous Oxide N2O Fertilisers, fossil fuel combustion and some synthetic chemical manufacturing processes are the main anthropogenic source of nitrous oxide. Nitrous oxide contributes to the destruction of the ozone layer and is also 250 times more effective than carbon dioxide as a greenhouse gas. Clearly then, we should constrain emissions of nitrous oxide. Estimates of sources of nitrous oxide are not very accurate but it is known, from measurements of Antarctic ice stores, that concentrations of N2O are currently 45% higher than during the last Glacial Maximum, suggesting that recent increases are due to human activities. |
|
Ozone O3 Normal oxygen molecules consist of two atoms of oxygen (O2). Ozone is a three-atom molecule (O3), and from an environmental point of view it is extremely important in two completely different ways |
|
|
1) Atmospheric ozone. Ozone is an effective greenhouse gas, particularly in the upper and middle troposphere. It is formed in the atmosphere, where a series of complex chemical reactions are catalysed by the action of sunlight on carbon monoxide (CO) and methane (CH4), nitrogen oxide radicals (NOx) and non methane hydrocarbons. Ozone in the upper atmosphere - the stratosphere, is part of an important naturally occurring shield around the Earth. The 'ozone layer' is involved in controlling the thermal structure of the atmosphere by absorbing incoming ultraviolet solar radiation and the outgoing longer-wavelength radiation from the Earth's surface. Ozone forms naturally in the stratosphere by the action of sunlight splitting an oxygen molecule (O2) into two separate oxygen atoms. These atoms then react with other oxygen molecules to produce ozone. The recognition of a substantial depletion in the concentration of stratosphere ozone was made in the 1970's and the first report of a 'hole' in the ozone layer was published by the British Antarctic Survey in 1985. Over Antarctica ozone depletion begins in September each year when the Antarctic polar vortex is isolated from other wind systems. Initially this ozone depletion was blamed on the large fleets of aircraft that emit nitrogen oxides (which play a role in stratospheric ozone depletion), but subsequently the main culprits were identified as chlorofluorocarbons (CFC's). |
|
|
2) Ground level ozone Ozone is a gas that is toxic to humans at concentrations as low as 1ppm. Low level ozone is a product of air pollution mainly from industry and cars. They release nitrogen dioxides (NOx) and sulphur dioxide (SO2) into the lower atmosphere and these react with sunlight to create photo-oxidants, of which ozone is the most dangerous. Ozone is a major pollutant in hot summers, particularly in large cities and industrial areas. Exposure to high levels of ozone is dangerous to humans, causing asthma attacks and other respiratory problems. The World Health Organisation recommends a limit of 76-100 ppb maximum exposure for one hour per day. Here the ground ozone pollution is a major ingredient in photochemical smog, a light haze can develop during hot day weather when pollutants from vehicles are trapped in still air. Many plants wilt or die when exposed to photochemical smogs, and people and animals suffer from irritation of the eyes and lungs. One way of controlling ozone smogs is to cut down on the emission of pollutant gases, particularly nitrogen oxide, from vehicle exhausts. New catalytic converters on vehicle exhausts are designed to reduce exhaust gas pollutants. |
|
| Other Ozone depleters: | |
| Halons (used in some fire extinguishers) | |
| Should have been phased out by January 1996 | |
| Methyl chloroform and carbon tetrachloride (both solvents) | |
| Should have been phased out by January 1990 | |
| Some CFC substitutes | |
| Should have been phased out by January 1990 | |
| The pesticide methyl bromide | |
| Consumption to be pegged at 1991 levels by 1995 | |